Techniques
-
![In Vivo Calcium Imaging with Miniature Microscopes]()
In Vivo Calcium Imaging with Miniature Microscopes
We use head-mounted miniature microendoscopes (Inscopix) to visualize the activity of individual cells within large neural ensembles during freely moving behavior. We use the activity dependent calcium sensor, GCamp, to label neurons in brain regions of interest either through viral-mediated gene delivery or through the use of transgenic mouse lines. We then implant gradient refractory index (GRIN) lenses into the mouse brain, which allows us to image the same ensemble of neurons longitudinally over several days and weeks. With this powerful technique, we can study neural network dynamics of genetically defined subpopulations of neurons in the brain of live and awake mice during the experience of stress or during cognitive or emotional behavioral tasks with unprecedented temporal resolution even in deep brain regions, such as the hippocampus.
-
![In Vivo Fiber Photometry]()
In Vivo Fiber Photometry
We use in vivo fiber photometry in combination with the activity dependent calcium sensor, GCamp, to measure neural population activity in the brain of freely moving mice. This technique allows us to measure activity in two different brain regions at the same time, to help us understand how different brain regions are implicated in the same behaviors, and how different brain regions react to the same environmental challenges or pharmacological treatments. Using dual color imaging, we can also visualize two different fluorophores within one brain region to study how different cell types within the same region are implicated in behavior. We also use this technique to image novel biosensors for serotonin in freely moving mice to understand how serotonin signaling in specific brain regions is affected by stress.
-
![Optogenetics]()
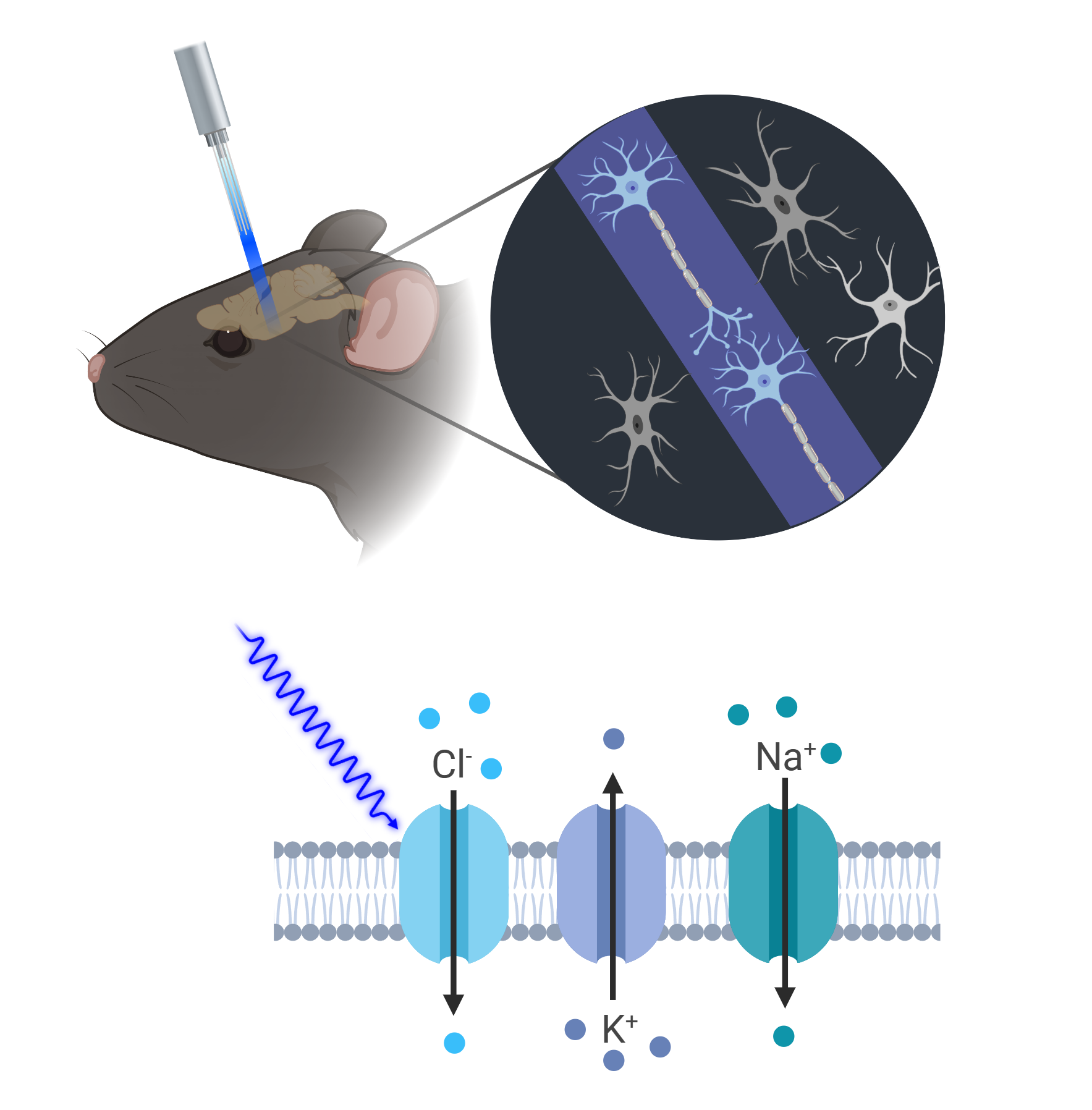
Optogenetics
We use optogenetics to assess ELA effects on fear generalization by selectively modulating distinct neuronal populations through precise temporal control over neuronal activity with light. This is achieved through the genetic introduction of light-sensitive ion channels, such as channelrhodopsin or halorhodopsin, enabling targeted activation or silencing of neurons via specific wavelengths of light. In our ELA studies, we use Cre mouse lines engineered to express these opsins selectively, facilitating the precise manipulation of defined neural circuits. This approach allows us to investigate how optogenetic stimulation can potentially counteract the effects of ELA on fear generalization, while precisely targeting specific serotonergic circuits.
-
![Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)]()
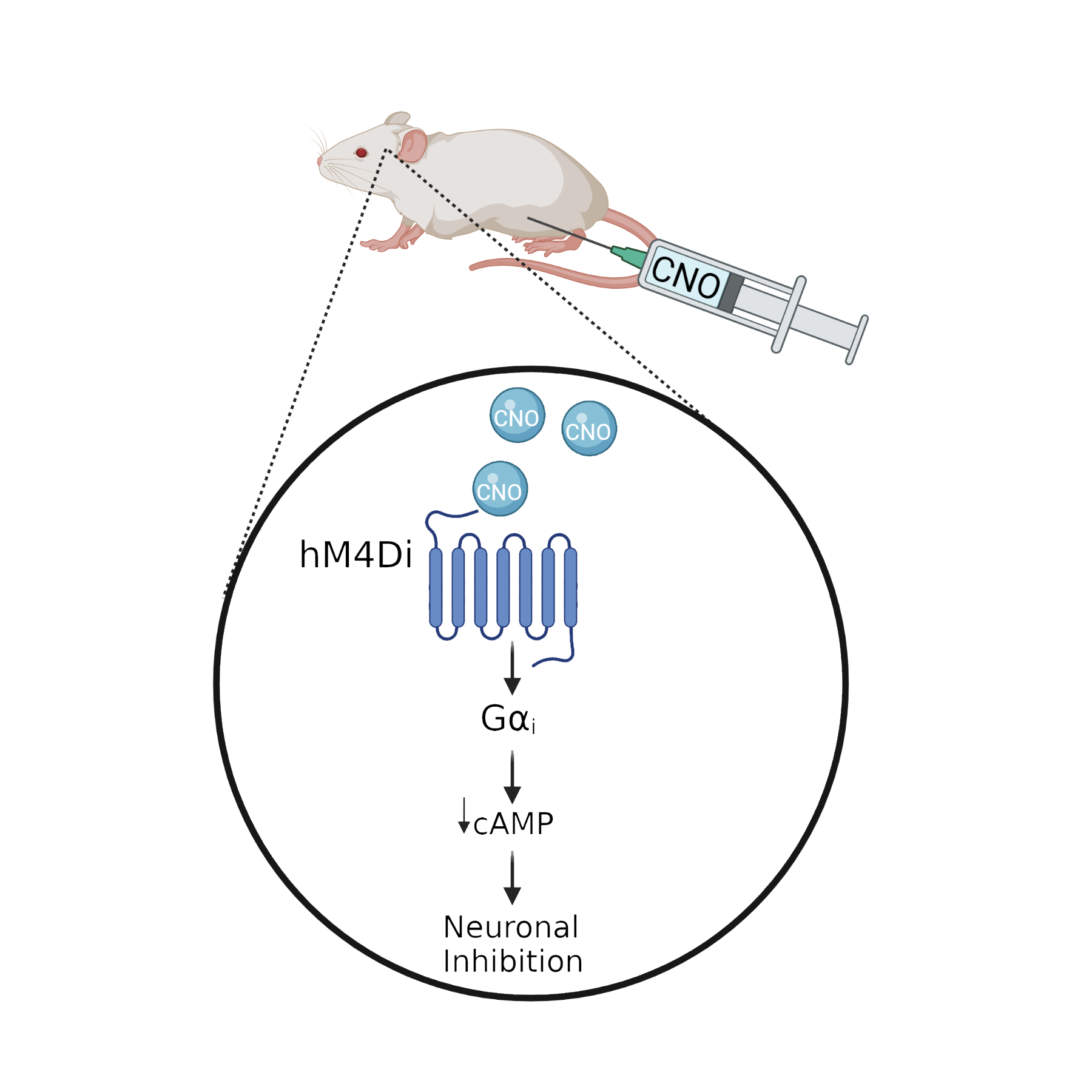
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)
DREADDs are genetically engineered human muscarinic receptors that no longer bind to their natural ligand, acetylcholine, but that are instead activated by the synthetic drug, clozapine-N-oxide (CNO). DREADDs can be inhibitory (hM4Di) or excitatory (hM3Dq) receptors that allow us to chronically silence or stimulate neural populations, respectively. We use viral-mediated delivery or transgenic mouse lines to express DREADDs in specific brain regions, in specific cell populations, or in specific projection neurons, to functionally manipulate the activity of these neurons during the experience of stress or during cognitive or emotional behavioral tasks. This approach allows us to investigate a functional role for specific cells and circuits for stress responses and behavior.
-
![Neural Circuit Manipulation]()
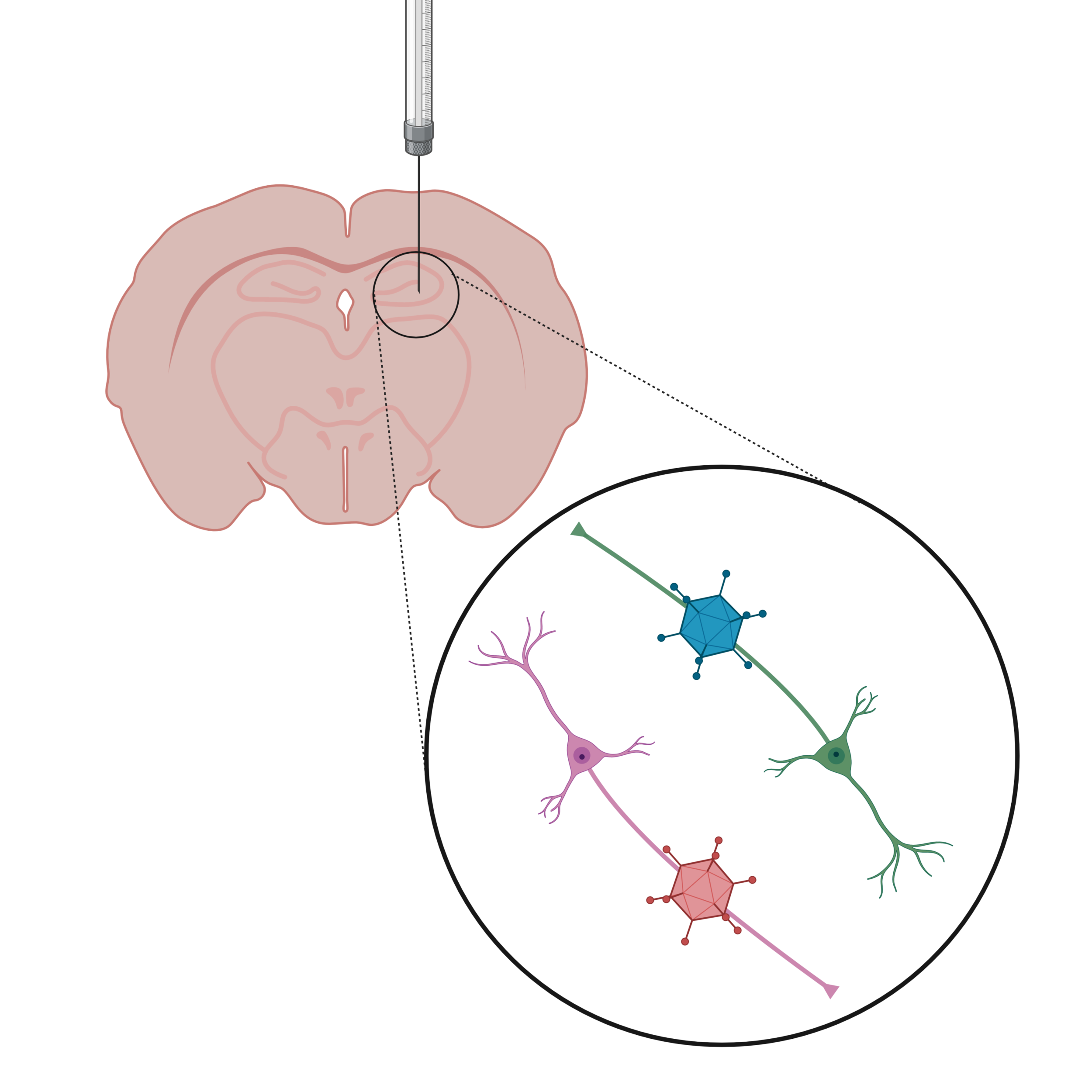
Neural Circuit Manipulation
We use anterograde and retrograde transported viruses in combination with the Cre/loxP system to label and functionally manipulate specific connections between different brain regions. Injecting a retrogradely transported Cre virus in the prefrontal cortex, and a Cre-dependent inhibitory DREADD virus in the hippocampus allows for the specific labeling of projection neurons from the hippocampus to the prefrontal cortex with the inhibitory hM4Di receptor. After 8 weeks of virus expression, CNO can be injected into the animal or directly delivered into the brain to specifically inhibit the activity of hippocampus-PFC projection neurons during the experience of stress or during behavioral tasks to investigate the functional role of these projection neurons for stress coping and behavior.
-
![Next Generation Sequencing]()
Next Generation Sequencing
We use next generation sequencing, such as RNA-seq or Whole Genome Bisulfite Sequencing (WGBS) to investigate gene expression and DNA methylation (DNAm) changes, respectively. These techniques allow us to gain a comprehensive understanding of how DNAm and gene expression in specific brain regions change in response to stress, how long-lasting these changes are, and if they can be transmitted from one generation to the next, and if we can find treatments to reverse these epigenetic and transcriptional responses to stress.
-
![]()
Rodent Stress Models
- Early Life Stress Model: the Limited Bedding and Nesting (LBN) model is used to study the effects of early life stress on brain development and behavior. In this model, during the critical early postnatal period, a dam and her pups are provided with insufficient nesting materials, creating an environment of scarcity and unpredictability. This limited environment induces significant stress in the mother, resulting in fragmented and inconsistent maternal care, which serves as a significant stressor for the developing pups. Exposure to LBN conditions during early life affects the maturation of neural circuits involved in emotional regulation, cognition and stress responses.
- Social Defeat Model: This model involves exposing a rodent to repeated encounters with a larger, more aggressive conspecific, leading to social defeat. The defeated mouse is subjected to physical and psychological stress, which closely mimics the effects of social stressors in humans, such as bullying or social exclusion. Social defeat induces long-lasting behavioral changes and also affects neurobiological processes, such as alterations in neurotransmitter systems, hormones levels neuroplasticity in key brain brain areas including amygdala, hippocampus, and prefrontal cortex.
- Chronic Corticosterone (CORT) Model: it is a widely used rodent model that simulates prolonged stress by administering corticosterone, the primary glucocorticoid stress hormone in rodents, over an extended period. This model is designed to mimic the effects of chronic stress, leading to alterations in brain regions such as the hippocampus, prefrontal cortex, and amygdala, which are involved in memory, cognition and emotional regulation. Chronic CORT exposure often results in depressive-like and anxiety-like behaviors in rodents, which as anhedonia and reduced exploratory behavior.
-
![Behavior Tests]()
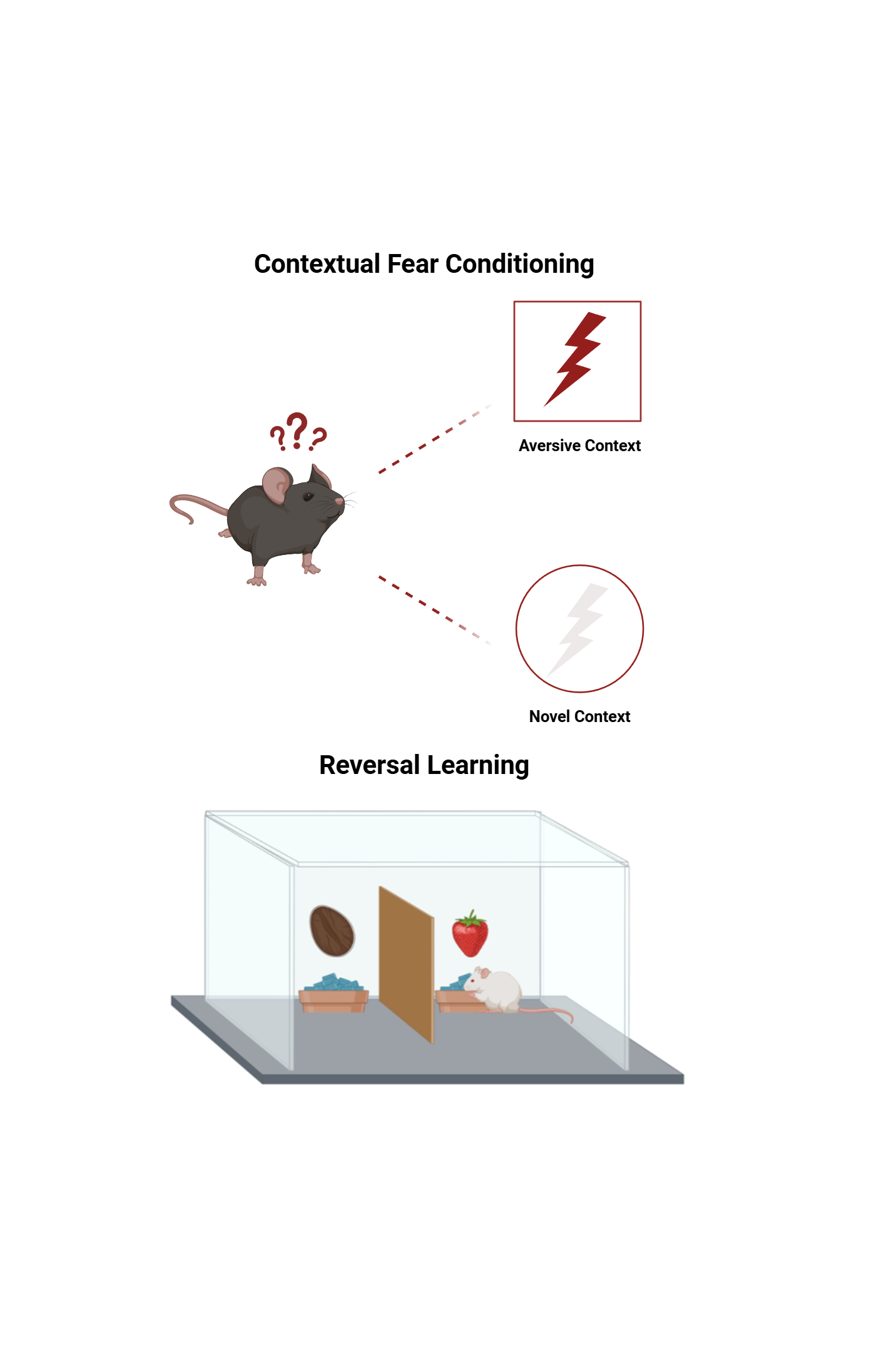
Behavior Tests
- Contextual Fear Conditioning: it is a widely used behavioral paradigm to study the neural mechanisms related to fear and anxiety. In this model, On day 1, rodents are placed in context A environment where they receive an aversive stimulus. On day 2, animals are re-exposed to the same context without the shock and 2 hours later mice are placed in a neutral context B. The rodent exhibits a fear response, typically measured by freezing behavior, which indicates memory recall of the stressful event and anxiety-like behaviors
- Cognitive Flexibility Tasks: Deficits in cognitive flexibility are often observed in neuropsychiatric disorders and these tasks are behavioral assessments used to evaluate an individual's ability to adapt their thinking and behavior in response to changing rules, environments, or contingencies. The 2-Choice Digging Task assesses reversal learning and attentional set shifting as complementary components of cognitive flexibility and animals must learn and then adapt to new rules or patterns to receive a reward.
-
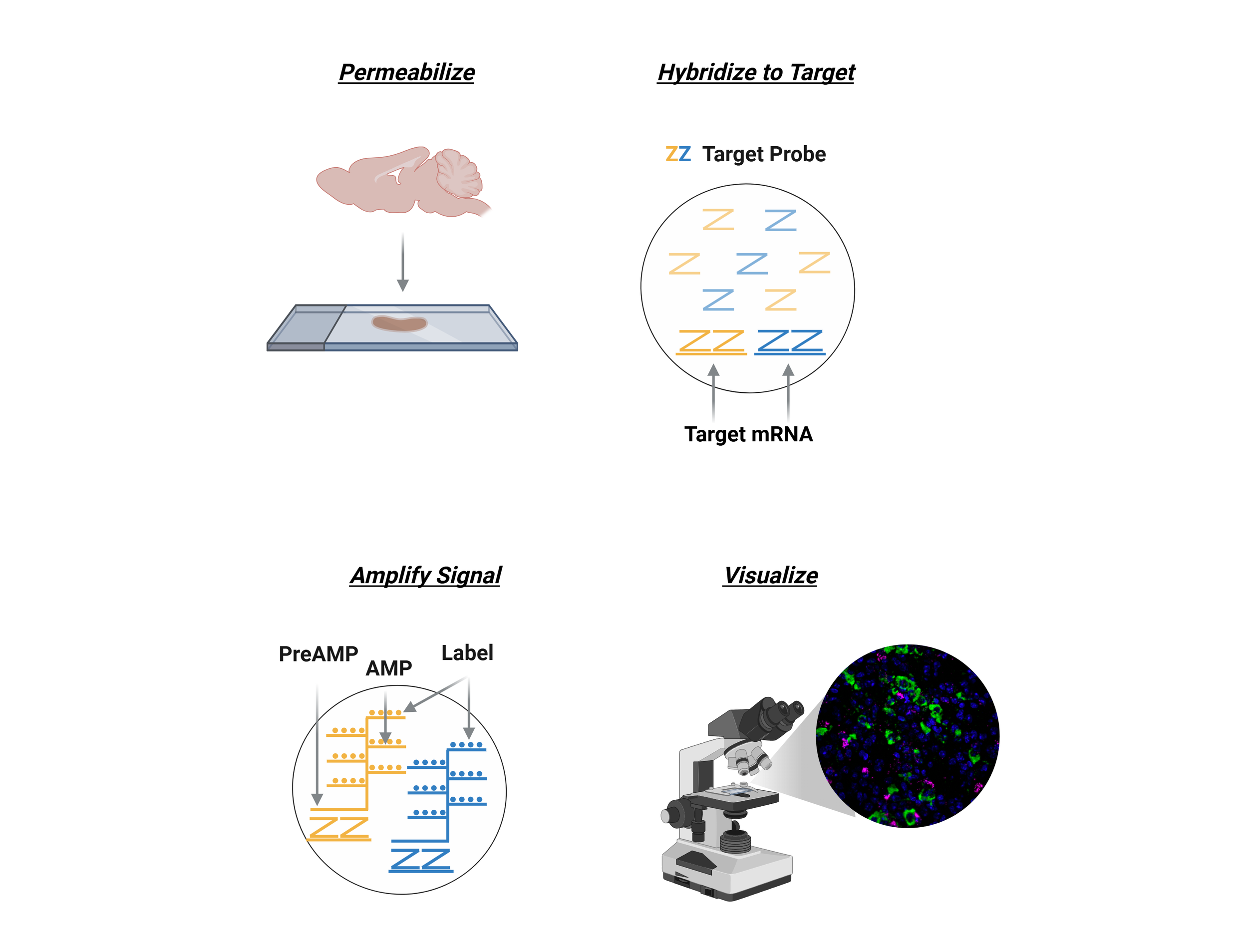
![RNAscope]()
RNAscope
RNAscope is an advanced in situ hybridization (ISH) technique that allows for the detection and visualization of RNA molecules in tissue samples or cultured cells with exceptional sensitivity and specificity. By utilizing a unique double Z-probe design, RNAscope amplifies the signal while minimizing background noise, enabling single-molecule RNA detection within individual cells. It supports multiplexing to visualize multiple RNA targets simultaneously, providing both quantitative and spatial information on gene expression. Compatible with various sample types, such as FFPE tissues, fresh frozen tissues, and cultured cells, RNAscope is widely used in fields like neuroscience, cancer research, and developmental biology to study gene expression, cellular interactions, and molecular mechanisms at the single-cell level.